
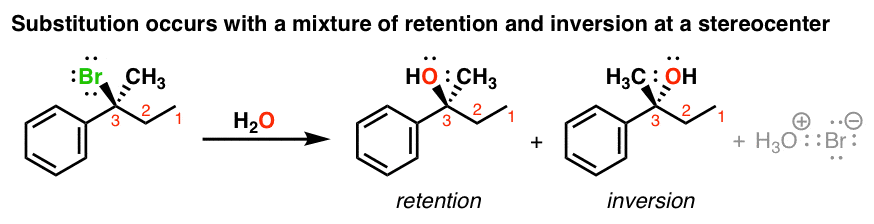
Mechanisms in which one elementary step is followed by another are very common. These elementary steps (also called elementary reactions) are almost always very simple ones involving one, two, or three chemical species which are classified, respectively, as unimolecular Elementary stepsĪ reaction mechanism must ultimately be understood as a "blow-by-blow" description of the molecular-level events whose sequence leads from reactants to products. Similarly, the presence of a catalyst can enable an alternative mechanism that greatly speeds up the rate of a reaction. For example, the dissociation of hydrogen bromide 2 HBr (g) → H 2 (g) + Br 2 (g) proceeds by different mechanisms (and follows different rate laws) when carried out in the dark ( thermal decomposition) and in the light ( photochemical decomposition). It is important to understand that the mechanism of a given net reaction may be different under different conditions.
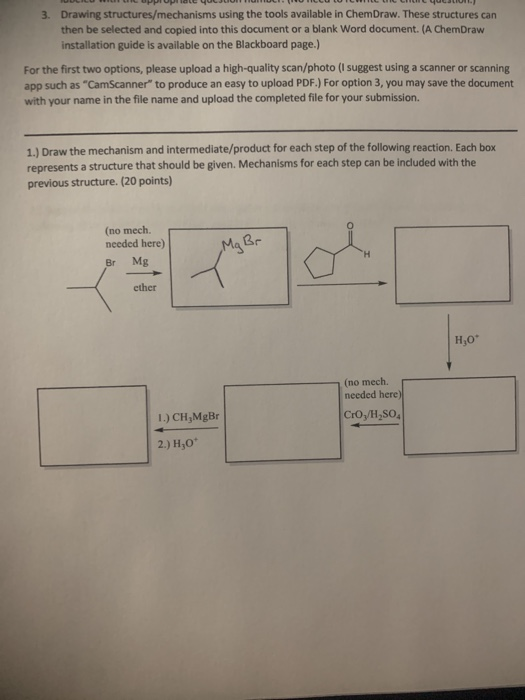
#Using chem draw for mechanisms series#
consists of a series of elementary steps (defined below) that can be written as chemical equations, and whose sum gives the net balanced reaction equation.
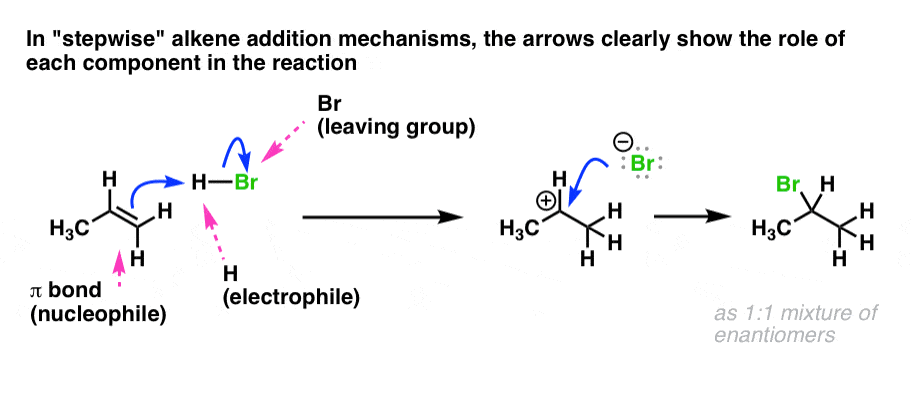
Note that the intermediate species NO 3 has only a transient existence and does not appear in the net equation. The mechanism of this reaction is believed to involve the following two elementary steps: For an example of a mechanism, consider the decomposition of nitrogen dioxide into nitric oxide and oxygen.


 0 kommentar(er)
0 kommentar(er)
